Introduction
 Some of our readers
that have examined the metallographic photomicrographs included
in our reports may have wondered about the process behind the images.
Metallography is a relatively involved practice that requires a
healthy blend of art and science. Some of our readers
that have examined the metallographic photomicrographs included
in our reports may have wondered about the process behind the images.
Metallography is a relatively involved practice that requires a
healthy blend of art and science.
Background
 Although the
term metallography was used as early as the 1700's, the practice
as we know it today is generally recognized as originating from
the pioneering work of the geologist Henry C. Sorby in the mid to
late 1800's. Beginning with his development of petrographic techniques
for the microscopic examination of geological samples, Sorby advanced
the science of iron and steel making in Sheffield, England by adapting
this approach for the study of meteoritic iron. Although the
term metallography was used as early as the 1700's, the practice
as we know it today is generally recognized as originating from
the pioneering work of the geologist Henry C. Sorby in the mid to
late 1800's. Beginning with his development of petrographic techniques
for the microscopic examination of geological samples, Sorby advanced
the science of iron and steel making in Sheffield, England by adapting
this approach for the study of meteoritic iron.
 Sorby's adaptation
of his petrographic sample preparation involved a long and painstaking
grinding and polishing procedure to incrementally remove the layer
of material deformed and distorted by the initial saw cut. Advances
in optics and metallurgy starting in the 1930's would finally build
upon Sorby's contribution to metallography and microscopic analysis
and bring us to the current state of the art. Sorby's adaptation
of his petrographic sample preparation involved a long and painstaking
grinding and polishing procedure to incrementally remove the layer
of material deformed and distorted by the initial saw cut. Advances
in optics and metallurgy starting in the 1930's would finally build
upon Sorby's contribution to metallography and microscopic analysis
and bring us to the current state of the art.
Applications
 Metallography
is the preferred technique for evaluating the internal structure
or microstructure of metallic materials. A metal's thermomechanical
history can be determined by examination of the microstructural
characteristics, such as prominent phases, grain size and texture.
This type of information is useful for failure analysis, process
development and quality control. Metallography
is the preferred technique for evaluating the internal structure
or microstructure of metallic materials. A metal's thermomechanical
history can be determined by examination of the microstructural
characteristics, such as prominent phases, grain size and texture.
This type of information is useful for failure analysis, process
development and quality control.
 Metallogrphy
is also gaining popularity for nonmetallic materials and composites. Metallogrphy
is also gaining popularity for nonmetallic materials and composites.
Process Outline
The basic steps of metallography are:
1. Sample Evaluation - Visual and optical microscopic examination
of the component to be analyzed is vital to determine the proper
location, size and orientation of the representative sample to be
prepared.
2. Sectioning - Diamond or water-cooled abrasive cutting wheels
are used to carefully remove the potential sample from the bulk
material with the least amount of thermal and mechanical damage.
3. Mounting - The sample is next mounted in themosetting compounds
such as Bakelite or clear acrylic, resulting in what is fondly referred
to as a hockey puck. The mounted sample is now well protected, easier
to handle and more stable.
A mounted sample Polishing
with abrasive Polishing
with abrasive
  
4. Grinding and Polishing - Wetted silicon carbide papers and diamond
films of decreasing grit size are next used to remove the damaged
surface material from the sectioning process and to access the area
of interest, such as the center of a corrosion pit. The final mirror-like
polish is usually achieved using 0.05 micron alumina slurry, although
chemical and electrochemical techniques are also available.
5. Examination - Optical examination of the as-polished sample with
a metallograph (an inverted stage, reflected light microscope) is
generally used for inclusion rating, dimensional measurement, fracture
analysis and fine crack detection. Various microscopic techniques
for increasing optical contrast, such as bright/dark field and polarization
are available for detailed and sometimes quantitative examination.
6. Etching - Acidic or alkaline solutions corrosive to the metal
being tested are next used to provide additional optical contrast
for phase identification and grain boundary delineation with the
Metallograph. For example, dilute nitric acid is often used for
general etching of carbon steels, whereas a picric acid mixture
helps to distinguish certain transitional phases. The etching solutions
can be applied to the sample various ways, including swabbing, soaking,
vaporizing and with electrical current.
A comparison of etched (left) and unetched
(right)
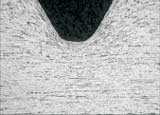  
7. Documentation - Digital photography of the microstructure is
now the standard image storage technology for metallography - much
more convenient than the Polaroid film used for decades. A digital
image can be easily transmitted, printed, archived and enhanced
for analysis.
 The field of
metallography is continuously expanding and will always strive to
incorporate the latest technology to keep pace with the needs of
Materials Scientists and Engineers. Many of the techniques outlined
above are also applicable to non-metallic materials such as plastics
and ceramics, illustrating the flexibility and broad range of this
interesting field. The field of
metallography is continuously expanding and will always strive to
incorporate the latest technology to keep pace with the needs of
Materials Scientists and Engineers. Many of the techniques outlined
above are also applicable to non-metallic materials such as plastics
and ceramics, illustrating the flexibility and broad range of this
interesting field.
 If you want to
see metallographic preparation in action, call us to arrange a visit. If you want to
see metallographic preparation in action, call us to arrange a visit.


 Stress corrosion
cracking (SCC), also called environmental cracking, is arguably
the most insidious form of metals failure in nature. In silence
and stealth, it usually progresses undetected until eventually something
detrimental occurs to shed light on its existence. It is important
to realize that virtually all engineering metals and alloys are
susceptible to this form of corrosion failure in selected chemical
media. However, this Achilles heel is different for the varying
families of metals. Some common examples are chloride cracking of
austenitic stainless steels, season cracking of copper alloys, caustic
embrittlement of steel, and sulfur cracking of nickel alloys. Stress corrosion
cracking (SCC), also called environmental cracking, is arguably
the most insidious form of metals failure in nature. In silence
and stealth, it usually progresses undetected until eventually something
detrimental occurs to shed light on its existence. It is important
to realize that virtually all engineering metals and alloys are
susceptible to this form of corrosion failure in selected chemical
media. However, this Achilles heel is different for the varying
families of metals. Some common examples are chloride cracking of
austenitic stainless steels, season cracking of copper alloys, caustic
embrittlement of steel, and sulfur cracking of nickel alloys.
Factors
 Although there
is much variability in the circumstances surrounding environmental
cracking, three common conditions do exist that are necessarily
required for SCC to be able to pounce on an unsuspecting metal or
alloy. If any of the three conditions are not met, cracking will
not occur. Those conditions are a chemical media capable of causing
cracking within a particular family of metals, elevated levels of
stress, and temperatures above a certain threshold level. Although there
is much variability in the circumstances surrounding environmental
cracking, three common conditions do exist that are necessarily
required for SCC to be able to pounce on an unsuspecting metal or
alloy. If any of the three conditions are not met, cracking will
not occur. Those conditions are a chemical media capable of causing
cracking within a particular family of metals, elevated levels of
stress, and temperatures above a certain threshold level.
Environment
 This is the most
important player in the stress corrosion cracking game since without
a chemical species capable of inducing cracking, SCC will never
occur. The reason why some media can crack certain metal families
and have no effect on others is not well understood. Usually, but
not always, reasonably high concentrations of an offending species
are necessary for SCC. The higher the concentration the sooner SCC
will occur, and in most cases water in some form is also necessary.
Unless a precedent exists, a confident prediction of what media
will crack what metals and alloys is not possible. Although similar
chemical species to known cracking media can be a guideline for
the possibility of cracking, testing of highly stressed samples
in a simulated environment is ultimately necessary. On a positive
note, corrosion literature over the last sixty or so years has compiled
numerous case histories of SCC. Just as various alloys in a particular
alloy family are similarly at risk, usually the guilty chemical
species also is part of an environmental family. An example is ammonia,
which is a known SCC agent for copper alloys. It can be present
as household ammonia, in agricultural fertilizers, bird dropping,
animal urine, insecticides and a host of other ammoniated chemical
compounds. Such literature information guides the corrosion engineer
in materials selection that avoids potential SCC situations. This is the most
important player in the stress corrosion cracking game since without
a chemical species capable of inducing cracking, SCC will never
occur. The reason why some media can crack certain metal families
and have no effect on others is not well understood. Usually, but
not always, reasonably high concentrations of an offending species
are necessary for SCC. The higher the concentration the sooner SCC
will occur, and in most cases water in some form is also necessary.
Unless a precedent exists, a confident prediction of what media
will crack what metals and alloys is not possible. Although similar
chemical species to known cracking media can be a guideline for
the possibility of cracking, testing of highly stressed samples
in a simulated environment is ultimately necessary. On a positive
note, corrosion literature over the last sixty or so years has compiled
numerous case histories of SCC. Just as various alloys in a particular
alloy family are similarly at risk, usually the guilty chemical
species also is part of an environmental family. An example is ammonia,
which is a known SCC agent for copper alloys. It can be present
as household ammonia, in agricultural fertilizers, bird dropping,
animal urine, insecticides and a host of other ammoniated chemical
compounds. Such literature information guides the corrosion engineer
in materials selection that avoids potential SCC situations.
Stress
 It has been well
documented that an incubation period precedes the initiation of
stress corrosion cracking, and the higher the stress levels in a
particular component the sooner the component will fail from SCC
in a media capable of causing SCC. Less quantifiable is the threshold
level of stress below which cracking will either not occur, or the
length of time it takes to begin cracking will be beyond the normal
lifespan of the component. However, the stress must be tensile in
nature and is not restricted to in service applied loads. Residual
stresses from casting, fabrication and welding play an important
additive role, and often in themselves are capable of imparting
surface tensile stress levels necessary for SCC to occur. It has been well
documented that an incubation period precedes the initiation of
stress corrosion cracking, and the higher the stress levels in a
particular component the sooner the component will fail from SCC
in a media capable of causing SCC. Less quantifiable is the threshold
level of stress below which cracking will either not occur, or the
length of time it takes to begin cracking will be beyond the normal
lifespan of the component. However, the stress must be tensile in
nature and is not restricted to in service applied loads. Residual
stresses from casting, fabrication and welding play an important
additive role, and often in themselves are capable of imparting
surface tensile stress levels necessary for SCC to occur.
Temperature
 A minimum temperature
must be reached before SCC occurs in susceptible environments, but
this is the most nebulous of the three conditions. A more realistic
approach is to develop a mindset that in general, increasing temperature,
like increasing stress levels, decreases the incubation time period
to the onslaught of cracking. Analogous to level of stress, a minimum
temperature usually exists below which cracking will either not
occur, or the length of time it takes to begin cracking is beyond
the normal lifespan of the component. For many situations this is
room temperatures. A minimum temperature
must be reached before SCC occurs in susceptible environments, but
this is the most nebulous of the three conditions. A more realistic
approach is to develop a mindset that in general, increasing temperature,
like increasing stress levels, decreases the incubation time period
to the onslaught of cracking. Analogous to level of stress, a minimum
temperature usually exists below which cracking will either not
occur, or the length of time it takes to begin cracking is beyond
the normal lifespan of the component. For many situations this is
room temperatures.
Metallurgical Examination
 A metallurgical
investigation into the cause of cracks in a component must differentiate
between other forms of cracking such as fatigue, corrosion assisted
fatigue, hydrogen embrittlement, progressive overload and impact
cracks. When viewed under a metallurgical microscope, stress corrosion
cracks are usually quite voluminous and have a unique, branched
morphology. The best analogy is of branches on a leafless tree with
the cracks initiating at the trunk and continuing to spread out
as they propagate away from the trunk. SCC can be both transgranular
(across the grains) or intergranular (along the grain boundaries),
a point that sometimes helps to identify the stress cracking agent.
Engery Dispersive Spectroscopy (EDS) in the Scanning Electron Microscope
(SEM) is used to determine the chemical elements present in and
around the cracks. This technique is often used to establish the
presence of a SCC species on a cracked component. A metallurgical
investigation into the cause of cracks in a component must differentiate
between other forms of cracking such as fatigue, corrosion assisted
fatigue, hydrogen embrittlement, progressive overload and impact
cracks. When viewed under a metallurgical microscope, stress corrosion
cracks are usually quite voluminous and have a unique, branched
morphology. The best analogy is of branches on a leafless tree with
the cracks initiating at the trunk and continuing to spread out
as they propagate away from the trunk. SCC can be both transgranular
(across the grains) or intergranular (along the grain boundaries),
a point that sometimes helps to identify the stress cracking agent.
Engery Dispersive Spectroscopy (EDS) in the Scanning Electron Microscope
(SEM) is used to determine the chemical elements present in and
around the cracks. This technique is often used to establish the
presence of a SCC species on a cracked component.
Case Study
 Because of the
extensive use of austenitic stainless steels in our society, and
the pervasiveness of chloride compounds, notably sodium chloride,
on this earth, chloride stress corrosion cracking of austenitic
stainless steels is by far the most important and common form of
SCC. Fortunately for the metallurgist, stainless steel is quite
resistant to uniform corrosive attack, and therefore chloride SCC
is usually not masked or obliterated by other forms of corrosion.
The chloride stress corrosion cracks in 200 and 300 series stainless
steels are distinctively very highly branched and almost always
transgranular. Because of the
extensive use of austenitic stainless steels in our society, and
the pervasiveness of chloride compounds, notably sodium chloride,
on this earth, chloride stress corrosion cracking of austenitic
stainless steels is by far the most important and common form of
SCC. Fortunately for the metallurgist, stainless steel is quite
resistant to uniform corrosive attack, and therefore chloride SCC
is usually not masked or obliterated by other forms of corrosion.
The chloride stress corrosion cracks in 200 and 300 series stainless
steels are distinctively very highly branched and almost always
transgranular.
Transgranular SCC of austenitic stainless
steel in a chloride environment

 The element
chlorine is easily detected using EDS. The element
chlorine is easily detected using EDS.
 Chloride SCC
in austenitic stainless steel is an excellent study of the three
conditions necessary for SCC to occur. 300 series stainless steels
are commonly used in chloride containing waters, cooking utensils
and even sea water without problems. For cracking to occur, the
most important condition is the need for the chloride salt to concentrate
to high levels through evaporation and remain so for a long period
of time. The temperature must be upwards of 100°F, and usually
residual stresses from manufacturing are enough for cracking to
occur. A personal example is a 60's vintage three-quart cooking
pot and lid, fabricated from 304 stainless steel sheet with a rolled
and folded formed lip on the lid. The pot has seen plenty of sodium
chloride salt from cooking over the years, especially this particular
size since it is ideal for boiling potatoes with the cover in place.
A white film usually forms at the edge of the lid during cooking.
The pot and the flat surfaces of the lid have never cracked since
both are washed after every use and thus the chloride salt does
not have a chance to concentrate. What did crack is the rolled and
folded formed lip on the lid. The fold provided enough of a crevice
so chlorides were able to have a place to concentrate and remain
for a long period of time. The temperature during cooking was well
above 100°F and plenty of residual stresses were present from
rolling and folding. Chloride SCC
in austenitic stainless steel is an excellent study of the three
conditions necessary for SCC to occur. 300 series stainless steels
are commonly used in chloride containing waters, cooking utensils
and even sea water without problems. For cracking to occur, the
most important condition is the need for the chloride salt to concentrate
to high levels through evaporation and remain so for a long period
of time. The temperature must be upwards of 100°F, and usually
residual stresses from manufacturing are enough for cracking to
occur. A personal example is a 60's vintage three-quart cooking
pot and lid, fabricated from 304 stainless steel sheet with a rolled
and folded formed lip on the lid. The pot has seen plenty of sodium
chloride salt from cooking over the years, especially this particular
size since it is ideal for boiling potatoes with the cover in place.
A white film usually forms at the edge of the lid during cooking.
The pot and the flat surfaces of the lid have never cracked since
both are washed after every use and thus the chloride salt does
not have a chance to concentrate. What did crack is the rolled and
folded formed lip on the lid. The fold provided enough of a crevice
so chlorides were able to have a place to concentrate and remain
for a long period of time. The temperature during cooking was well
above 100°F and plenty of residual stresses were present from
rolling and folding.
Prevention
 In materials
selection for a particular application, the most successful and
often the least difficult SCC mitigation approach is to select a
metal or alloy this is not susceptible under the environmental conditions
it will see. If this is not possible due to design or economic reasons,
modifying the environment so SCC will not occur is also highly successful,
but often this is the most difficult approach to achieve. If neither
of these is possible, reducing the surface tensile stresses is next
line of attack. This can be accomplished by reducing stress concentrators
in the initial design, stress relief annealing to reduce residual
stresses, shot peening to provide a thin surface layer of compressive
rather than tensile stress, and beefing up the component to reduce
the effect of applied stresses. A less desirable approach is to
isolate the component from the offending environment by use of a
coating or plating, which risks breaches in the barrier layer either
inherent to the layer or that could occur over time, and thus may
not be a reliable preventative. In materials
selection for a particular application, the most successful and
often the least difficult SCC mitigation approach is to select a
metal or alloy this is not susceptible under the environmental conditions
it will see. If this is not possible due to design or economic reasons,
modifying the environment so SCC will not occur is also highly successful,
but often this is the most difficult approach to achieve. If neither
of these is possible, reducing the surface tensile stresses is next
line of attack. This can be accomplished by reducing stress concentrators
in the initial design, stress relief annealing to reduce residual
stresses, shot peening to provide a thin surface layer of compressive
rather than tensile stress, and beefing up the component to reduce
the effect of applied stresses. A less desirable approach is to
isolate the component from the offending environment by use of a
coating or plating, which risks breaches in the barrier layer either
inherent to the layer or that could occur over time, and thus may
not be a reliable preventative.
Summary
 The first step
to avoiding stress corrosion cracking is to determine through literature
sources or in consultation with us if the environments your component
will experience have a historical possibility of causing SCC in
the alloys you have selected. Be conservative, and if the answer
is yes, evaluate the actual operating conditions and the physical
state your component is in to assess the feasibility of cracking
actually occurring. If the answer still remains that SCC is possible,
implement the preventative steps outlined in the article to reduce
or even eliminate the risk of SCC. The first step
to avoiding stress corrosion cracking is to determine through literature
sources or in consultation with us if the environments your component
will experience have a historical possibility of causing SCC in
the alloys you have selected. Be conservative, and if the answer
is yes, evaluate the actual operating conditions and the physical
state your component is in to assess the feasibility of cracking
actually occurring. If the answer still remains that SCC is possible,
implement the preventative steps outlined in the article to reduce
or even eliminate the risk of SCC.

 The scanning
electron microscope (SEM) is a powerful tool, capable of magnifications
up to 180,000 times. It allows us to reveal information which is
critical to metallurgical investigations, such as fracture modes
and surface characteristics. The scanning
electron microscope (SEM) is a powerful tool, capable of magnifications
up to 180,000 times. It allows us to reveal information which is
critical to metallurgical investigations, such as fracture modes
and surface characteristics.
 The SEM can also
be fun to play with, because it allows one to view the surface of
anything at high magnification with great depth of field. All of
us have been amazed by the pictures of various insect parts, especially
the eye of a fly. The SEM can also
be fun to play with, because it allows one to view the surface of
anything at high magnification with great depth of field. All of
us have been amazed by the pictures of various insect parts, especially
the eye of a fly.
 In this issue's
contest, we take a look at an object on the SEM that should be familiar
to all of you, and ask you to guess what it is. In past issues,
we have looked a kitchen mold, lava rock, various fabric fibers
and the tip of a fishing hook. This time, there are four images
representing two similar items taken at two different magnifications.
If you still think this is too difficult, here is a hint: George
Washington. In this issue's
contest, we take a look at an object on the SEM that should be familiar
to all of you, and ask you to guess what it is. In past issues,
we have looked a kitchen mold, lava rock, various fabric fibers
and the tip of a fishing hook. This time, there are four images
representing two similar items taken at two different magnifications.
If you still think this is too difficult, here is a hint: George
Washington.
 Expertise in
dendrochronology may not be useful to win this contest, but may
get you Headed down the right path. Expertise in
dendrochronology may not be useful to win this contest, but may
get you Headed down the right path.
   
 15X 15X  15X 15X
  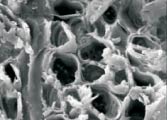
 250X 250X 500X 500X
 Please fax, mail
or e-mail us (don't call) with your answer. We will draw a winner
from all correct entries received by November 1. The correct answer
and the winner will be published in the next Of Materials Interest
issue. Please fax, mail
or e-mail us (don't call) with your answer. We will draw a winner
from all correct entries received by November 1. The correct answer
and the winner will be published in the next Of Materials Interest
issue.
 The prize is
a $50 gift certificate to a restaurant of your choice and a MEi
polo shirt, so put on your thinking caps and your appetites. The prize is
a $50 gift certificate to a restaurant of your choice and a MEi
polo shirt, so put on your thinking caps and your appetites.
|